The US FDA’s final rule on Requirements for Additional Traceability Records for Certain Foods (Food Traceability Final Rule) establishes traceability recordkeeping requirements beyond those in existing regulations for companies who manufacture, process, pack, or hold foods included on the Food Traceability List (FTL).
The new requirements identified in the final rule will allow for faster identification and rapid removal of potentially contaminated food from the market, resulting in fewer foodborne illnesses and/or deaths.
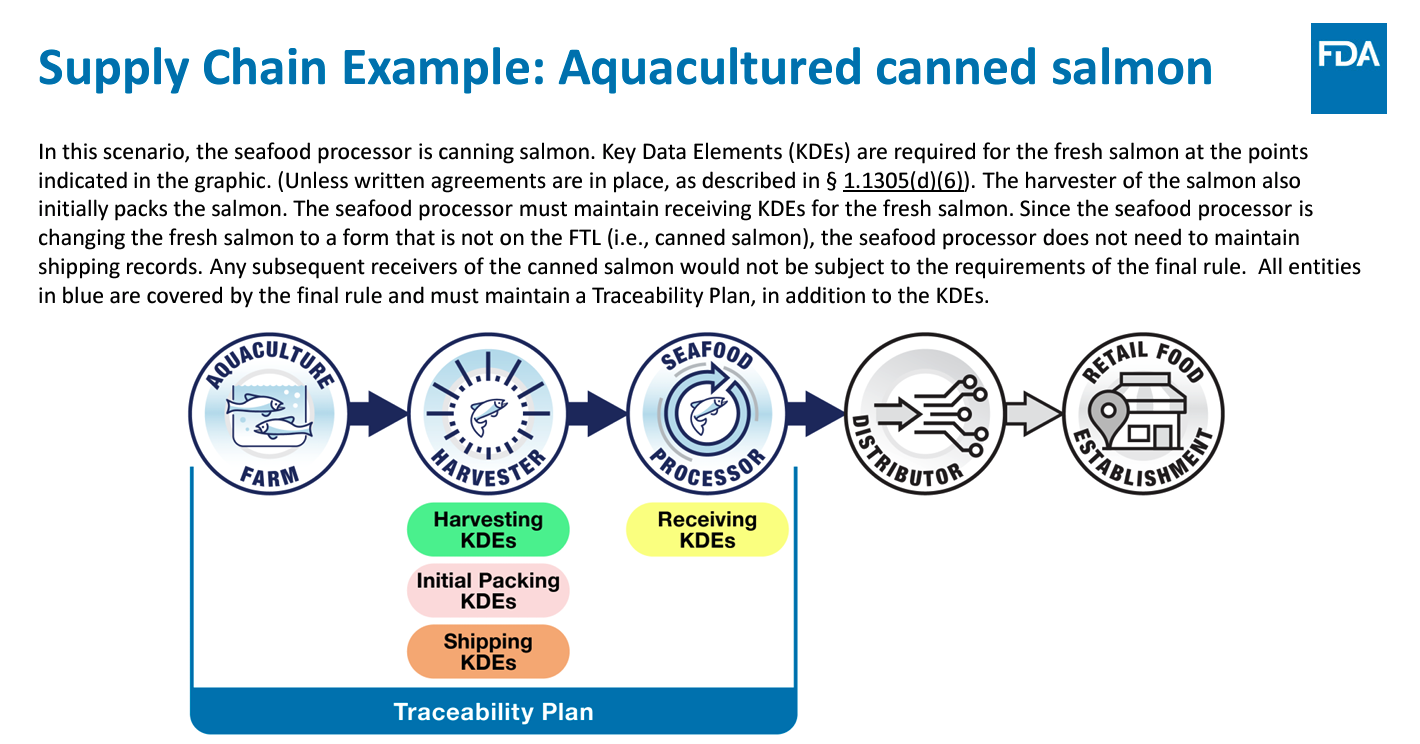
At the core of this rule is a requirement to maintain records containing Key Data Elements (KDEs) associated with specific Critical Tracking Events (CTEs).
The Key Factors also refer to Cooling (active temperature reduction using hydro cooling, icing, forced air cooling, vacuum cooling, or a similar process) and Shipping, when an electronic sortable spreadsheet containing relevant traceability information must be provided to the FDA within 24 hours of a request. Keeping records of temperature and humidity will be required in order to be compatible with the new guidelines.
The final rule covers US companies as well as other companies which produce food for U.S. consumption.
For example, the attached image demonstrates a possible supply chain process for Wild Caught Tuna in its journey to the client, with multiple touchpoints to monitor along the way.
Fourtec’s products aim to assist in ensuring food safety, food shelf life, and the reduction of food loss and waste by offering temperature and humidity monitoring solutions for the food and food processing market, including for Caterers, Ready Meal Kits and Last Mile delivery companies.
Fourtec’s DataSuite software and FourtecLite Android app enable data monitoring & analysis, as well as lifetime data storage that can be reviewed in graphs, tables, and logger statistics, printed or exported into Excel or CSV files.

